Nickel(II) chloride hexahydrate, 99.95% (metals basis), Each
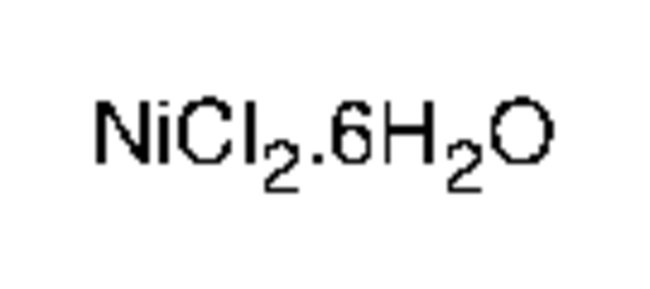
|
|
Details:
Nickel(II) chloride is used in electroplating and as a catalyst for organic conversions, for example in chemo-selective thioacetalization of aldehydes. In combination with lithium aluminum hydride, it serves as a reducing agent for alkenes, alkynes, and organic halides; it can cleave N-O bond and open epoxides. It is used to prepare a wide array of complexes since water ligands can be easily replaced by amines, thioether, ammonia, and thiolates. It is a precursor to several nickel-phosphine complexes, such as bis(triphenylphosphine)nickel(II) chloride, which are used in alkyne trimerizations, carbonylations, and as catalysts in organic reactions such as Suzuki-Miyaura cross coupling reactions as an alternative to palladium(0) catalysts. It is the precursor to acetylacetonate complex of Ni, used for producing 1,5-cyclooctadiene complex, an important reagent in organonickel chemistry. It can be used to prepare the sandwich compound nickelocene through dimethoxyethane complex of nickel chloride.
Additional Information
| SKU | 10025777 |
|---|---|
| UOM | Each |
| UNSPSC | 12352300 |
| Manufacturer Part Number | 053131A1 |
| CAS Number | 7791-20-0 |
| Is Hazardous | Yes |
| HS Code | 2827350000 |
|---|---|
| UN Number | UN 3288 |
| Proper Shipping Name | Nickel(II) chloride hexahydrate |
| Packaging Group | PG III |
| Commodity Code | 595 |
| DG or HZ | DG |
| Hazardous Class | 6.1 |
| Label |  |
| Molecular Formula | Cl2H2NiO |
| EC Number | 616-576-7 |
| HIN | 90 |
| Hazard Statement | H301+H331-H315-H317-H334-H341-H350i-H360D-H372-H410 |
| GHS | GHS06,GHS08,GHS09 |
| GHS (Pictogram) |    |
| Hazard Code | T,N,Xi |
| Signal Word | Danger |

