GGA3 Mouse anti-Human, Unlabeled, Clone: 8, BD, Mouse Monoclonal Antibody, Each
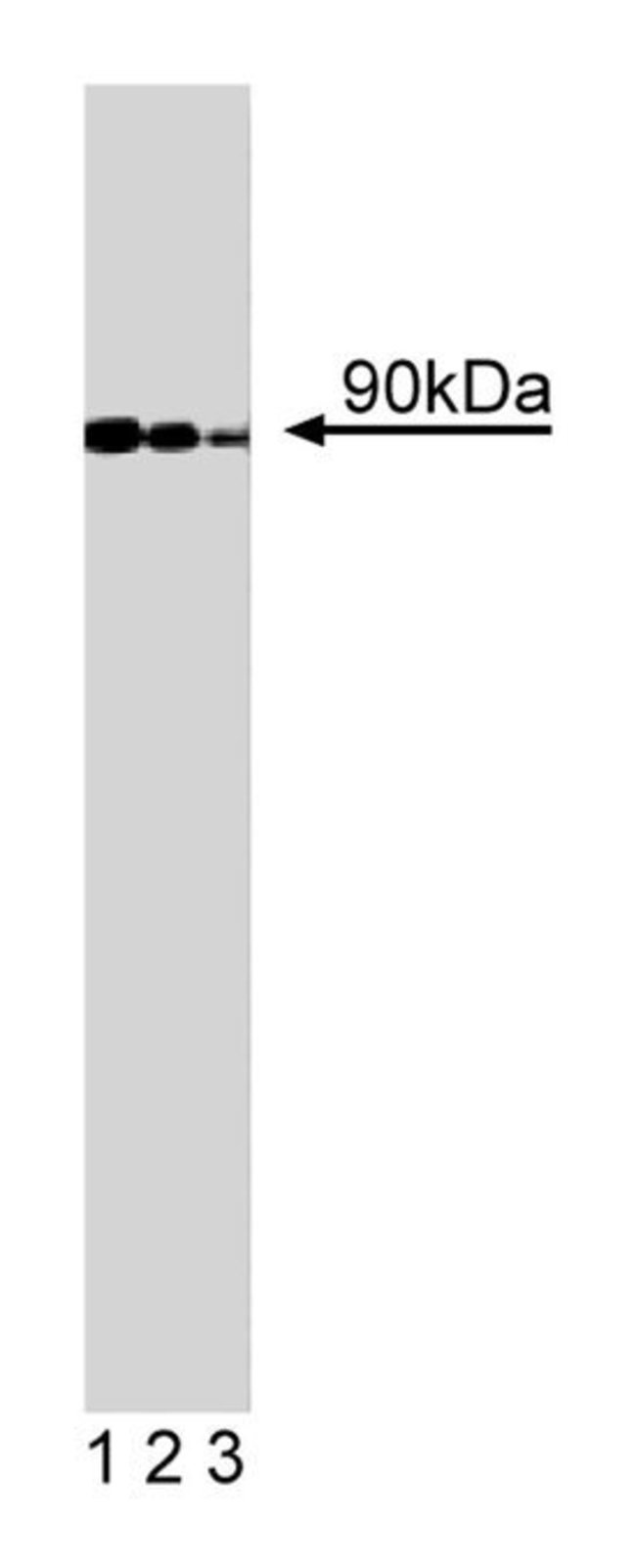
Details:
The ADP-ribosylation factors (ARFs) are a family of small GTPases in the ARF superfamily that include ARFs and ARF-like (ARLs) proteins. At least six ARFs have been identified in humans: ARF1, ARF2, ARF3, ARF4, ARF5, and ARF6. ARFs are involved in intravesicular acidification and fusion of microsomal vesicles, endosome fusion, nuclear membrane assembly, and formation of clathrin-coated vesicles. GGAs are ARF-binding proteins that act as adaptor coat proteins associated with the Golgi complex. GGA1, GGA2, and GGA3 are homologous proteins that contain N-terminal VHS domains, a GGA and TOM homology region (GAT), and a C-terminal region homologous to the ear domain of γ-adaptins. GGAs co-localize with Golgi markers in the TGN, and GGA3 is found present in coated vesicles and buds associated with the TGN. The GAT domain of GGA3 facilitates ARF1 binding, Golgi localization, and dissociation from ARF-regulated membranes. The C-terminal region of GGAs bind to MAP1A and rabaptin-5, which are binding partners of γ-adaptins. Overexpression of GGAs alters the distribution of markers normally found in the TGN. Thus, GGAs are ARF binding proteins that regulate vesicle dynamics in the TGN.Immunofluorescence, Western Blotting
Additional Information
| SKU | 10135549 |
|---|---|
| UOM | Each |
| UNSPSC | 12352203 |
| Manufacturer Part Number | 612310 |

