CD45 Mouse, Unlabeled, Clone: 69, BD, Mouse Monoclonal Antibody, Each
Details:
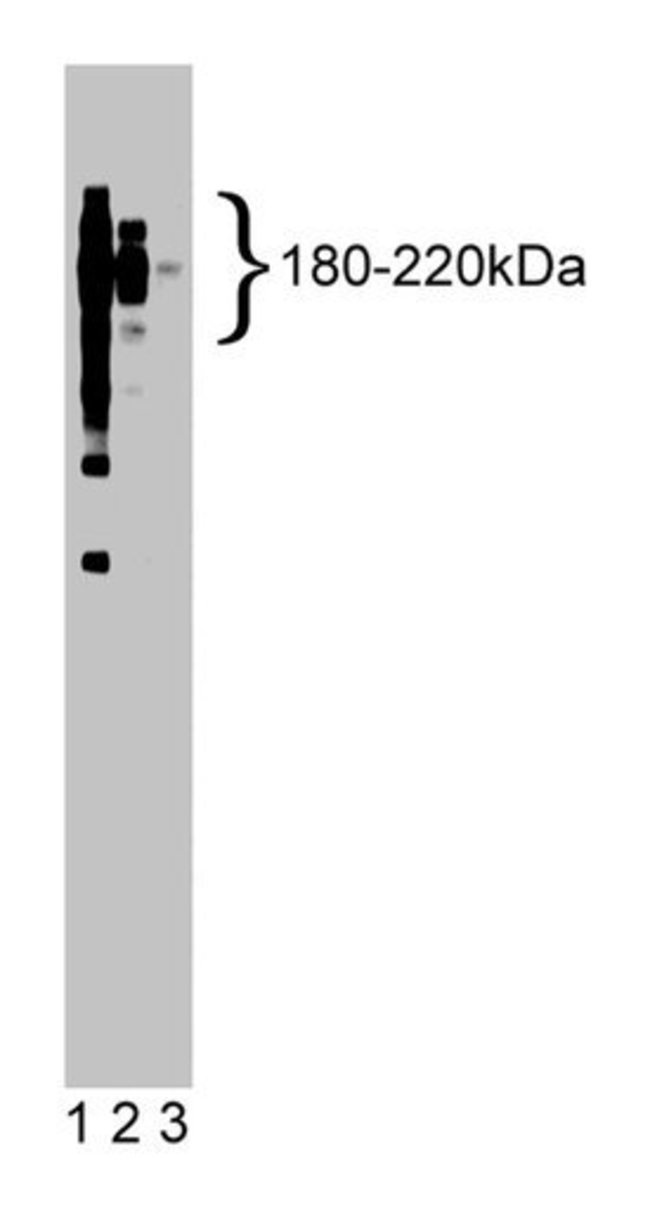
CD45 (also known as leukocyte common antigen, GP180, or T200) is found on all hematopoietic cells except those of red cell origin. The N-terminal domain is a large glycosylated extracellular region of variable length (390-542 amino acids) derived by the alternative splicing of at least three exons (4, 5, or 6). This variation accounts for the different isoforms of CD45 (180-220 kDa). The remainder of the molecule includes a short transmembrane region followed by a large highly conserved cytoplasmic domain of 702 residues. The CD45 cytoplasmic domain possesses an intrinsic protein tyrosine phosphatase (PTPase) activity. This region contains two PTPase domains (domains I and II) which share 40% homology. Both domains are required for optimal PTPase activity and show significant homology with other receptor-like and cytoplasmic PTPases. CD45 plays a critical role in antigen receptor-induced responses of T and B cells. In B cells, CD45 can exist as part of a larger multisubunit complex composed of several tyrosine phosphorylated proteins, including the Src-family PTK, Lyn, or it can exist as the membrane immunoglobulin-associated MB-1/B29 heterodimer. In T cells, CD45 associates with and dephosphorylates both lck and fyn. In addition, CD45 can associate with membrane proteins involved in T cell activation, such as CD2, Thy-1, TCR, and CD26.
Additional Information
| SKU | 10134881 |
|---|---|
| UOM | Each |
| UNSPSC | 12352203 |
| Manufacturer Part Number | 610266 |

